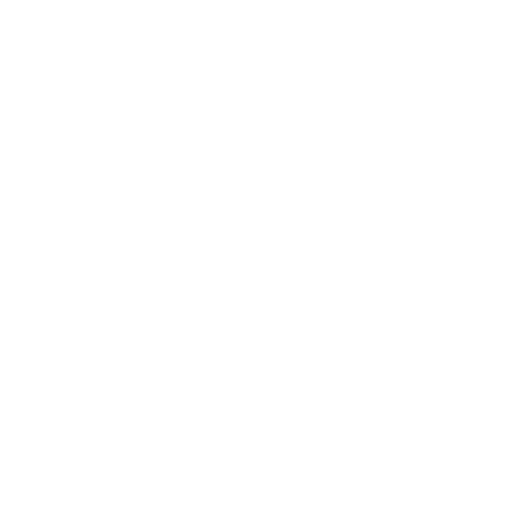Targeted Intradermal IL-17 Inhibition
A New Therapeutic Paradigm for Mild Plaque Psoriasis
Advancing Precision Immunotherapy
Offering new hope to millions of patients.
Scinai Immunotherapeutics is developing a targeted, intralesional IL-17A/F VHH antibody fragment (NanoAb) designed to deliver biologic-level efficacy and specificity without systemic exposure - finally addressing the massive unmet need of the mild-to-moderate psoriasis population.

The Unmet Need
Mild to moderate plaque psoriasis: the unspoken Underserved Population
Mild-to-moderate plaque psoriasis is a large, underserved population. Despite limited body coverage, disease in sensitive areas- such as the face, genitals, scalp, palms, and soles -can profoundly affect quality of life.
15.7
million
in 7 major markets
(US, EU5 and Japan);
80-90%
with plaque psoriasis
THE GAP IN TODAY’S TREATMENT LANDSCAPE
Current treatments for mild plaque psoriasis rely on topical therapies and phototherapy, which are limited by local side effects such as irritation and skin atrophy, particularly in sensitive or visible areas. These treatments are also inconvenient, requiring daily application or frequent clinic visits, leading to poor adherence and suboptimal outcomes.
As a result, many patients remain dissatisfied with available options.
Biologic therapies are reserved for moderate-to-severe plaque psoriasis. While these therapies offer high efficacy, they carry systemic immunosuppression and high costs, making them inappropriate for patients with limited disease.
Payers and clinicians continue to seek safe, localized, and affordable alternatives that improve adherence and patient satisfaction.

Our Solution
INTRADERMAL DELIVERY OF AN ANTI-IL-17A/F VHH
How it works?
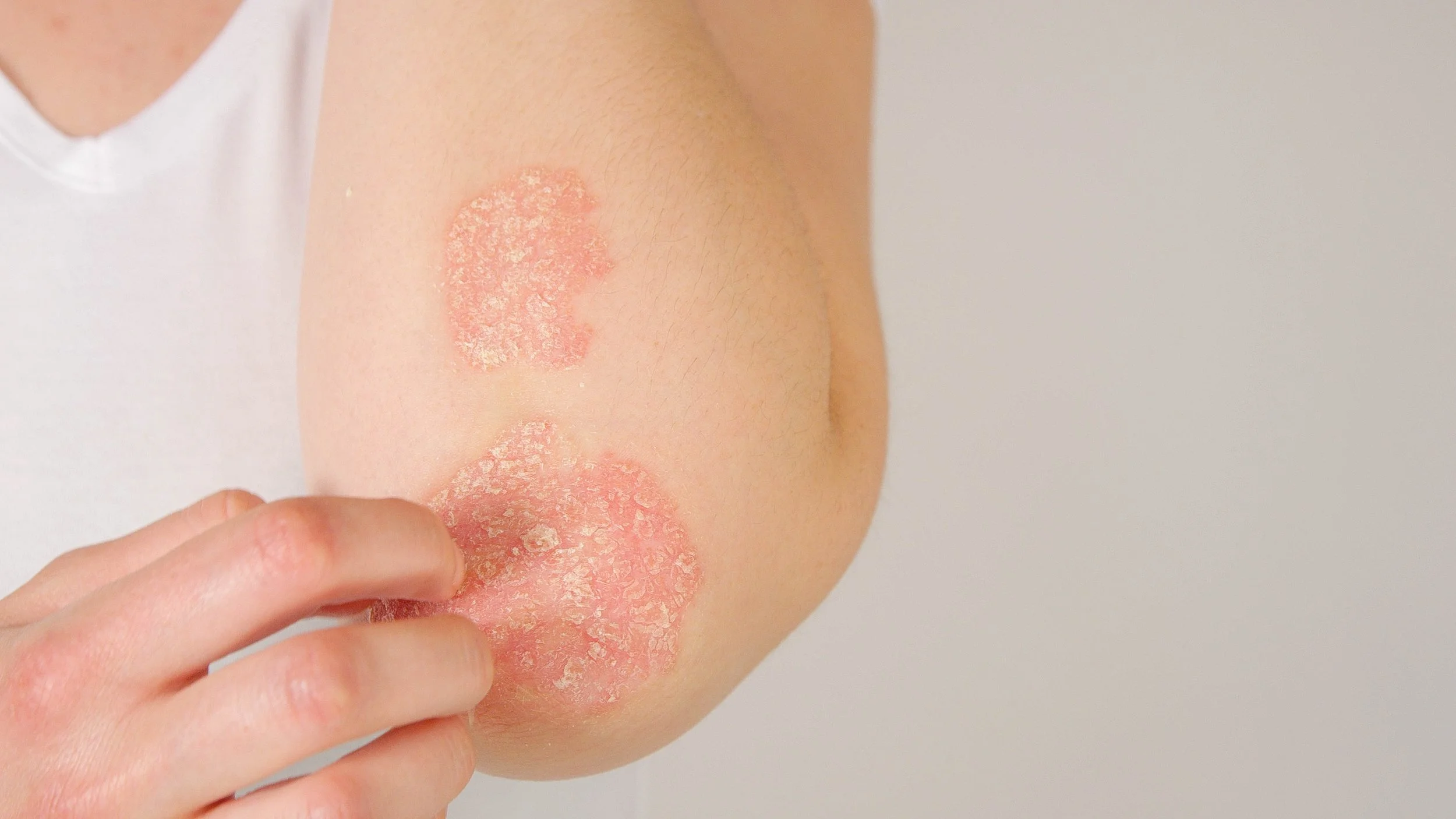
Precise Intradermal Delivery Into the Lesion
Utilizes a mesotherapy-style painless multi mini-injections to deliver the NanoAb formulated in a sustained release directly into psoriatic tissue while minimizing systemic exposure.
Targeted IL-17 Pathway Blockade at the Source
The NanoAb locally neutralizes both IL-17A and IL-17F within the lesion, disrupting the inflammatory cascade at the site of disease activity.
One Short and Painless treatment Every Few Months
A fast, low-volume procedure designed for infrequent dosing and improved patient compliance.
Treatment Benefits: Biologic-level efficacy and specificity, low systemic exposure combined with patient-friendly delivery.
Convenient Delivery
A quick, in-office session under 15 minutes, needed only once every three months.
Precise Targeting
Directly reaches lesions up to 10% BSA, including sensitive or hard-to-treat areas.
Accessible & Affordable
Designed for mild-to-moderate patients and expected to cost less than newer topicals and injectable biologics.
High Efficacy, Low Risk
Matches biologic-level effectiveness while minimizing systemic immunosuppression and side effects.

Pre-Clinical Results
Preclinical data show the anti-IL-17 NanoAb blocks IL-17A/F, disrupting the local psoriatic inflammatory cascade.
Intralesional delivery reduces key inflammatory markers with efficacy comparable to leading therapies.
A sustained-release formulation provides effects lasting ~3 months, while minimal systemic exposure and no observed adverse effects support a safe, durable profile.
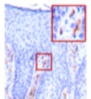
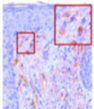
Ex-Vivo Proof of Concept: NanoAbs Shown to Block
IL-17 cytokines
In vivo proof of concept:
healed lesions in the Secukinumab and NanoAb treatments.
IL-17A expression (red frame)
Observed in the negative control
Blocked by NanoAb, Seckukinumab and the study’s positive control (Dexamethasone)
IL-17F expression (red frame)
Observed in the negative control and in Secukinumab Blocked only by the NanoAb, and the study’s positive control (Dexamethasone)
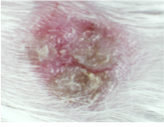
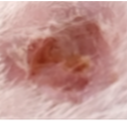
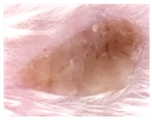
At study termination, samples from mice transplanted with skin from the same donor show scales and psoriatic appearance in the Negative control and healed lesions in the Secukinumab and NanoAb treatments.
Negative control
Secukinumab
Nanoab
(once a week)
Future Plans & Timeline
Program: IL-17A/F
Indication: Local intradermal treatment of psoriasis
Licensor: Max Planck Society and the University Medical Center Gottingen
Patent: PCT issued in 2023
Ex-vivo PoC- proving MoA in skin tissue
Done
First in-vivo PoC – efficacy in live psoriatic tissue
Done
Second in-vivo PoC –
duration of effect
H1 2026
Toxicology
H2 20 26
Contact us
Get in touch to explore partnership opportunities and consult our CDMO specialists