Smarter Bioprocess Development with the AMBR 250 Fermenter System
Transforming Bioprocess Development with the AMBR 250 Fermenter System at Scinai BioServices
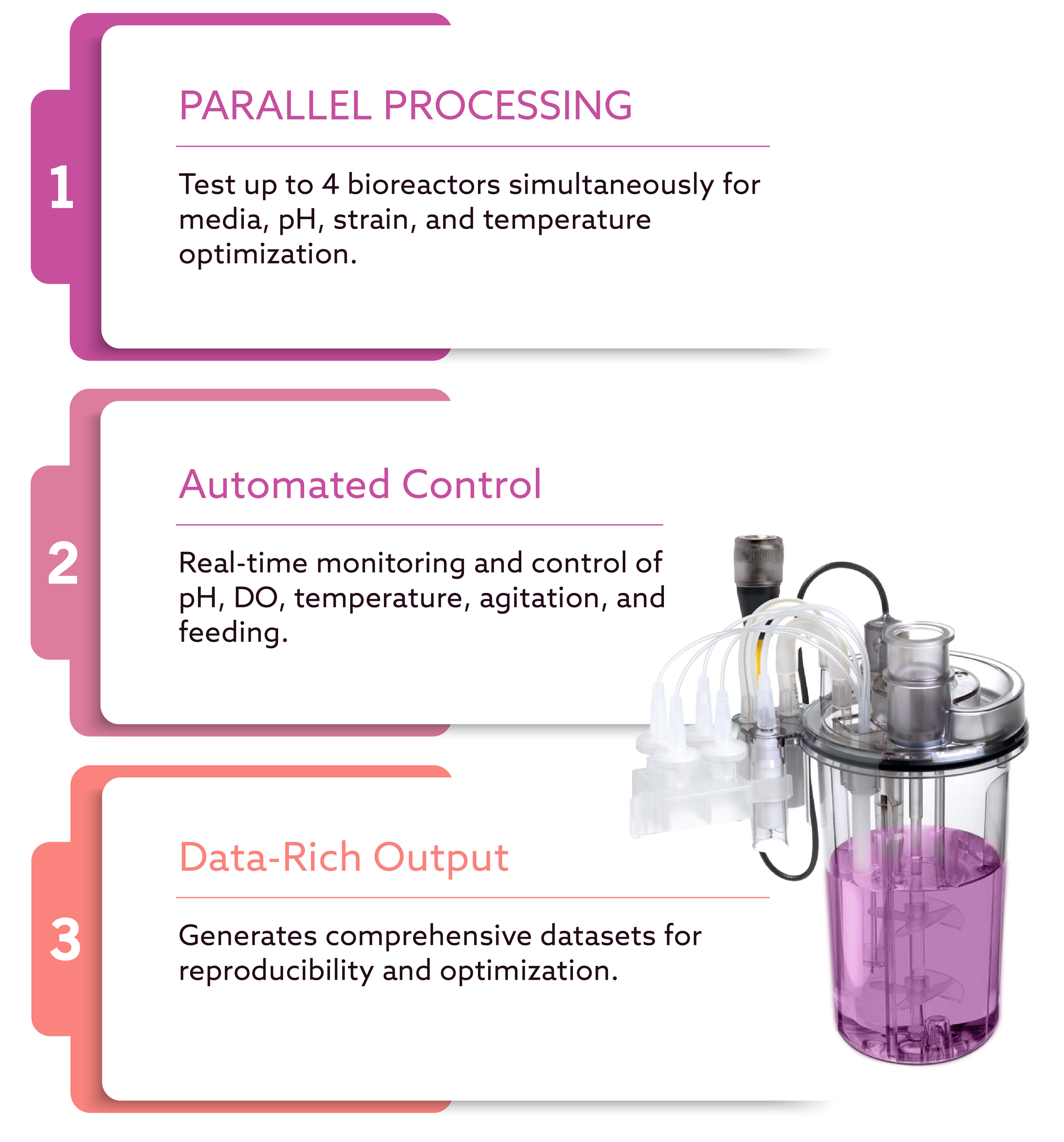
High-Throughput Fermentation and Process Optimization
At Scinai BioServices, one of the technologies that enables us to accelerate fermentation development is the Sartorius AMBR 250, a highly automated mini-bioreactor system designed for rapid, data-rich microbial and cell-culture process development.
In this blog, we highlight what the AMBR system is, how it works, and why it has become a transformative platform in our CDMO workflows.
What Is the AMBR 250 Fermentor System?
The AMBR 250 (Advanced Micro Fermenter) is a high-throughput, automated mini-bioreactor platform widely used in biotechnology and pharmaceutical R&D. Designed for small working volumes (up to 250 mL), it allows researchers to:
Test multiple fermentation or cell-culture conditions in parallel
Reduce material and reagent use
Generate statistically meaningful data early in development
With the ability to mimic larger bioreactor behavior, AMBR 250 serves as a powerful scale-down model that supports reliable scale-up into pilot and production systems.
How the AMBR 250 Works
1. High-Throughput Parallel Processing
The system supports up to 4 parallel mini-bioreactors, enabling rapid screening of variables such as:
Media composition
Strain selection
Temperature and pH settings
Feeding strategies
This parallel design dramatically shortens development time compared to conventional 1–10 L fermenters.
2. Automation for Reliability and Reproducibility
AMBR automates critical control parameters:
pH
Dissolved oxygen (DO)
Agitation
Temperature
Feeding strategies
Automation reduces manual intervention, minimizes variability, and ensures smoother, more consistent experimental runs.
3. Integrated Monitoring and Real-Time Control
Built-in sensors continuously track and adjust:
DO
pH
Temperature
Agitation
These feedback control loops allow precise environmental regulation — essential for high-quality process development.
4. Data-Rich Experimentation
AMBR generates a large volume of high-resolution data suitable for:
Statistical analysis
Process characterization
DoE (Design of Experiments) studies
Multivariate optimization
This data-rich environment supports faster, smarter decision-making.
5. Reliable Scale-Down and Predictable Scale-Up
AMBR 250 accurately simulates hydrodynamics and mass transfer characteristics found in larger bioreactors, making it a dependable platform for:
Early-stage feasibility work
Mid-stage optimization
Later-stage scale-up planning
This increases confidence when transferring methods to pilot and production volumes.
Why Scientists Trust the AMBR 250
The AMBR technology is widely valued in the bioprocess community for its:
High-throughput capabilities
Low working volumes and reduced material costs
Automated controls for strong reproducibility
Real-time monitoring and feedback systems
Data-rich outputs ideal for DoE and modeling
Predictive scale-down performance
Reduced labor and faster turnaround
Applicability to both cell culture and microbial fermentation
Together, these features make AMBR one of the most effective platforms for early-stage process development.
Driving Bioprocess Innovation at Scinai
At Scinai BioServices, the AMBR 250 is a central component of our fermentation development workflow. We use it to:
Rapidly evaluate media and strain combinations
Screen pH, temperature, DO, and feeding strategies
Conduct high-quality DoE studies with fewer resources
Build statistically validated, scalable processes
Seamlessly transfer optimized conditions into larger bioreactor for GMP production
By integrating AMBR into our CDMO processes, we save time, reduce costs, and provide our partners with a faster route to scalable, production-ready fermentation systems.
A Word from Zohar Gadri, GMP Development Team Leader
“The AMBR fermentor system allows us to generate high-quality data faster and with far greater consistency — it’s a real game-changer for our CDMO workflows. Its automation, throughput, and reliable scale-down capabilities give us the confidence to optimize quickly and scale predictably.”
Zohar Gadri, R&D Technology Expert & GMP Development Team Leader, Scinai BioServices