Elevating Drug Development.
Every Step of the Way
About Scinai Immunotherapeutics
nasdaq: scni
Scinai is a biopharmaceutical company focused on developing inflammation and immunology (I&I) biological products and on providing CDMO services through its Scinai Bio-services business unit.

Scinai Research and Development
Inflammatory & IMMUNOLOGY
Therapeutics Pipeline
Scinai is focused on developing, manufacturing, and commercializing innovative inflammation and immunology (I&I) biological products primarily for the treatment of autoimmune and infectious diseases.
Our Alpaca-derived nanosized antibodies (nanoAbs), also known as VHH-antibodies, address diseases with large unmet medical needs and attractive commercial opportunities, such as psoriasis and asthma.
Diverse drug platform
Proven target molecules
& mechanism of action
Addressing large markets with unmet needs
Saving costs & improving patient compliance
DEVELOPED IN collaboration with WORLD LEADERS IN NANOAB
discovery & OPTIMIZATION
Professor Dr.
Dirk Görlich
Professor & Director at Max Planck Institute for Multidisciplinary Sciences
2024 Louis-Jeantet
Prize & 2022 WLA Prize
Professor Dr. Matthias Dobbelstein
Max Planck Institute Fellow, Professor and Department Head at the University of Göttingen
Scinai’s NanoAbs are developed in collaboration with the world-renowned Max Planck Institute for Multidisciplinary Sciences and University Medical Center Göttingen, Germany.
Our scientific partners are leaders in designing and optimizing NanoAbs.
Scinai CDMO Bioservices
END -TO-END DRUG DEVELOPMENT
AND MANUFACTURING
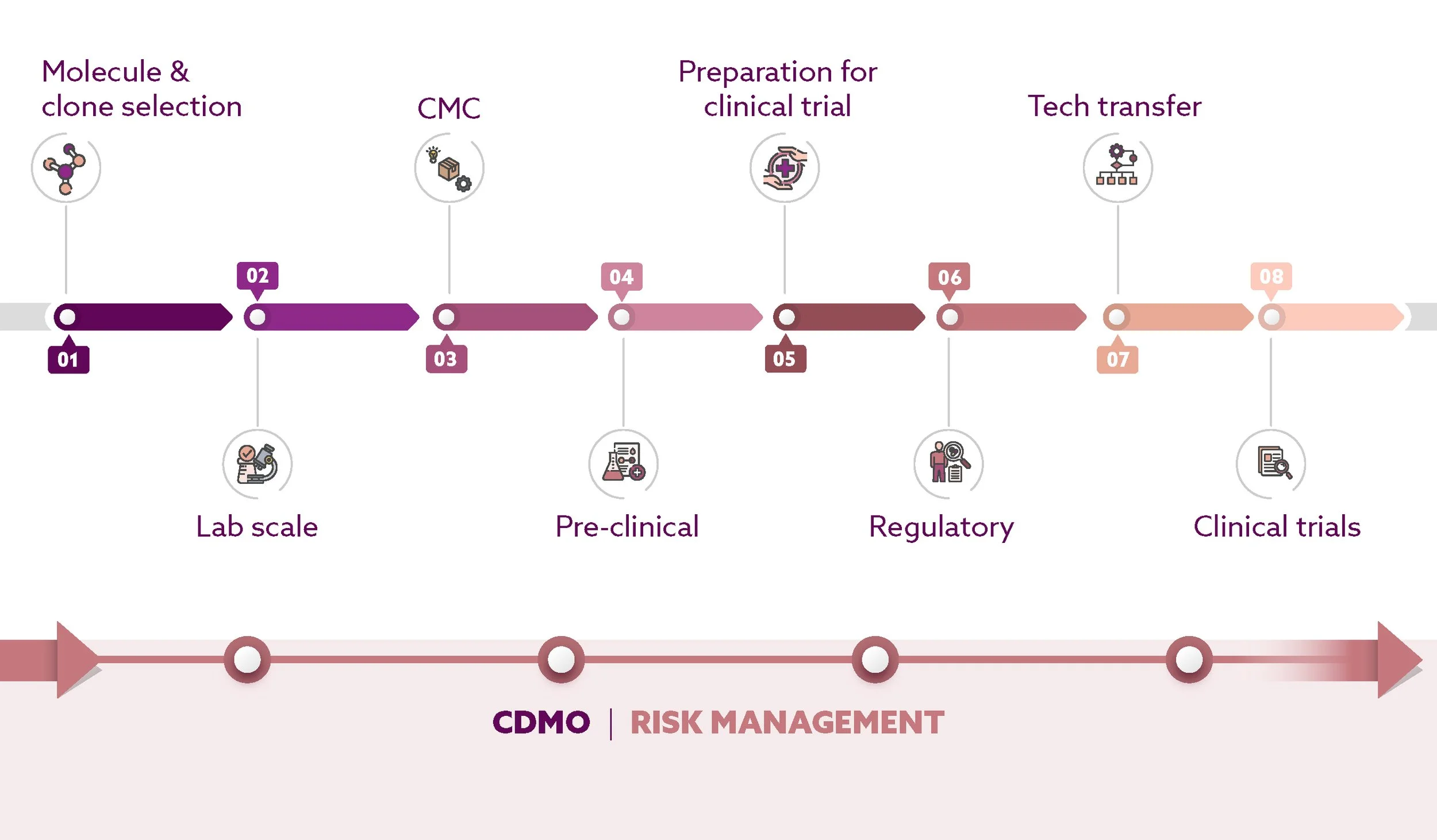
Our boutique biologics CDMO is designed to support biotech innovators through every stage of drug development - from early research to Phase 3 manufacturing.
Backed by over 20 years of experience and a state-of-the-art cGMP facility, we deliver flexible, agile, and cost-effective solutions that combine scientific innovation with process excellence for reliable, high-quality product delivery.
Scinai CDMO Bioservices
END -TO-END DRUG DEVELOPMENT
AND MANUFACTURING
MOLECULE & CLONE SELECTION
LAB SCALE
CMC
PRE-CLINICAL
PREPARATION FOR CLINICAL TRIAL
REGULATORY
TECH TRANSFER
CLINICAL TRIALS
Our boutique biologics CDMO is designed to support biotech innovators through every stage of drug development - from early research to Phase 3 manufacturing.
Backed by over 20 years of experience and a state-of-the-art cGMP facility, we deliver flexible, agile, and cost-effective solutions that combine scientific innovation with process excellence for reliable, high-quality product delivery.
cGMP Manufacturing
Our high-end manufacturing suites are designed to meet FDA and EMA cGMP standards and have successfully passed European QP (EMA) and Israeli MoH audits for Phase 3 clinical trial production.
Analytical
Methods
Development
Our experienced team of scientists utilizes advanced analytical technologies to ensure precise and reliable product characterization. We provide comprehensive analytical method development for in-process control, release testing, and detailed analysis of recombinant proteins.
Process Development
& Scale-Up
With extensive expertise in recombinant protein development, Scinai delivers cost-effective, flexible, and scalable process development and scale-up solutions. Our experts guide clients through each stage of research and development, helping to minimize risks and accelerate progress toward clinical success.
Trusted by…

Contact us
Get in touch to explore partnership opportunities and consult our CDMO specialists